USA - Recommendations for Clinical Laboratory Improvement Amendments of 1988 (CLIA) Waiver Applications for Manufacturers of In Vitro Diagnostic Devices - RIS.WORLD

What does CLIA-88 mean? - Definition of CLIA-88 - CLIA-88 stands for Clinical Laboratory Improvement Amendments of 1988. By AcronymsAndSlang.com

Smithers Receives CLIA Certification For New Jersey Pharmaceutical Development Services Facility - Smithers

CMSGov on Twitter: "Want updates on the CLIA program? Subscribe to the new CMS DCLIQ #CLIACOMMS: https://t.co/z4Toklx5G2 https://t.co/A1LZUl9Obb" / Twitter
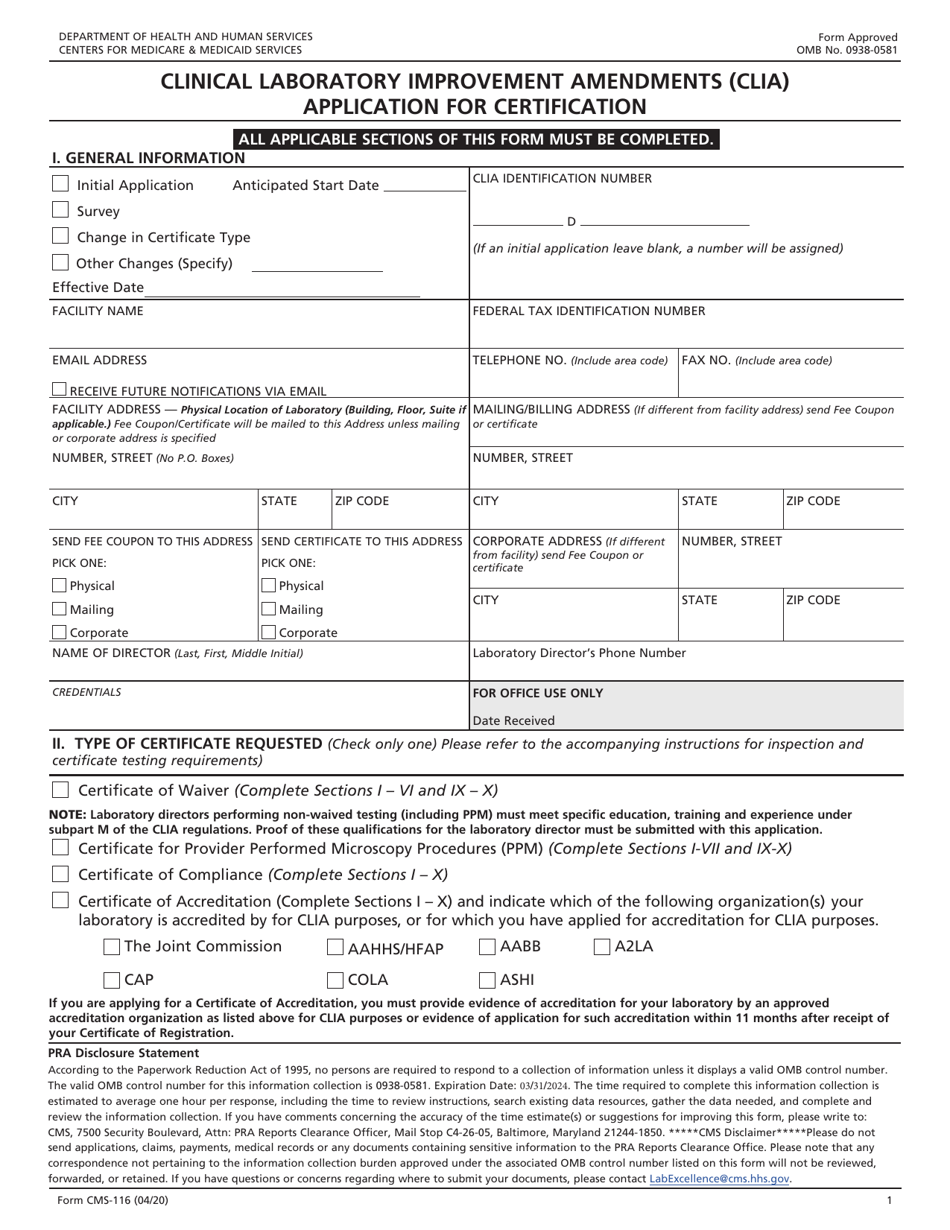
Form CMS-116 Download Fillable PDF or Fill Online Clinical Laboratory Improvement Amendments (Clia) Application for Certification | Templateroller

Improving patient outcomes through personalized ca Our Genetic Tests E-Metab (Estrogen) Panel DMEX Genotype Panel (P450) Warfarin GenoSTAT Clopidogrel GenoSTAT Oncology Panel MTHFR Panel Test Directory Newsroom About Us Contact Us ...

New FDA Guidance Issued for Clinical Laboratory Improvement Amendments (CLIA) Waiver Applications | AZBio