Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine
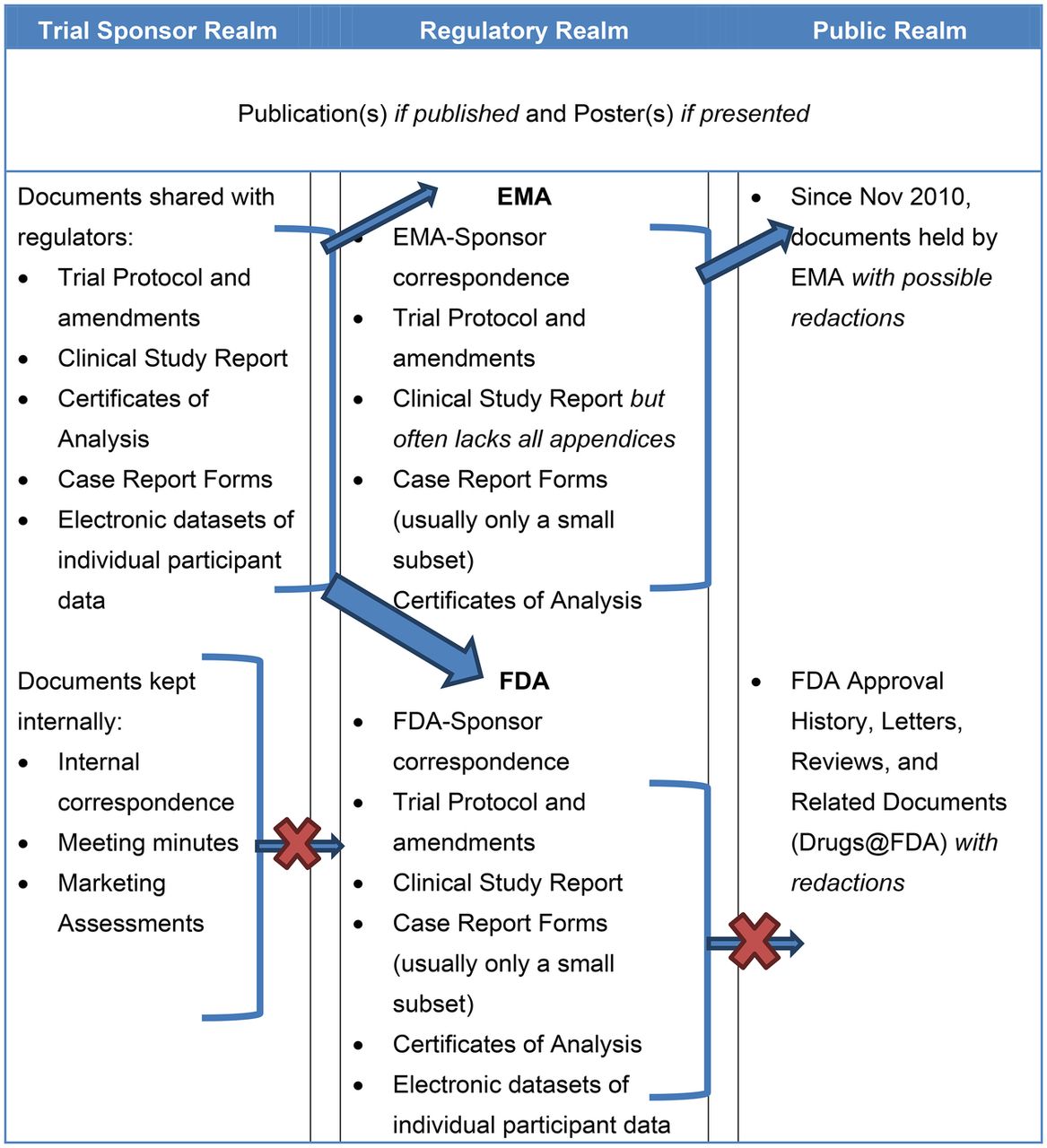
Clinical study reports of randomised controlled trials: an exploratory review of previously confidential industry reports | BMJ Open