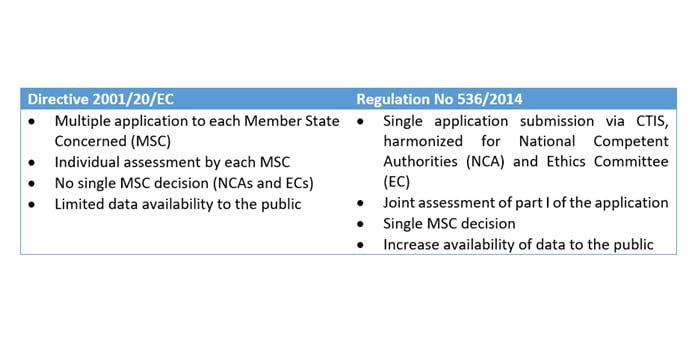
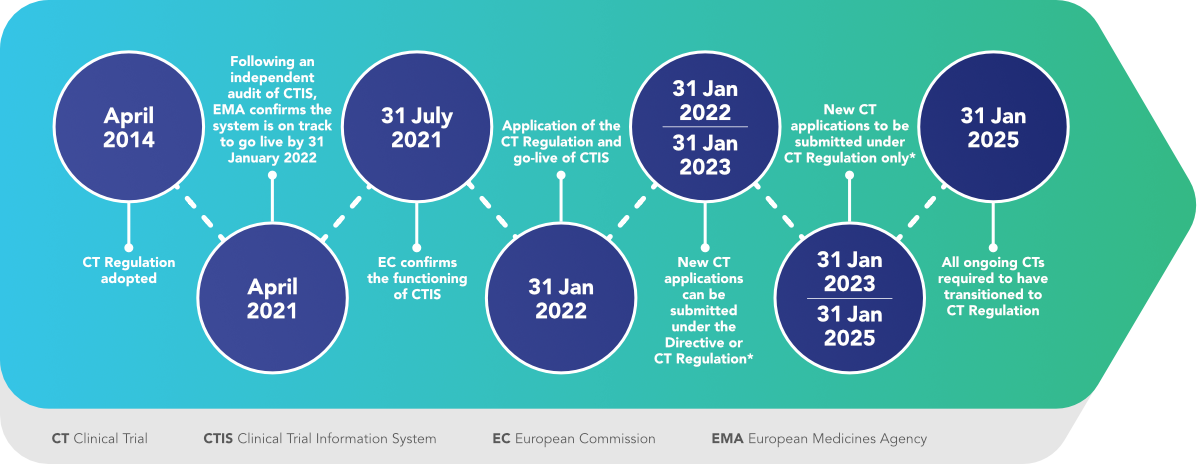
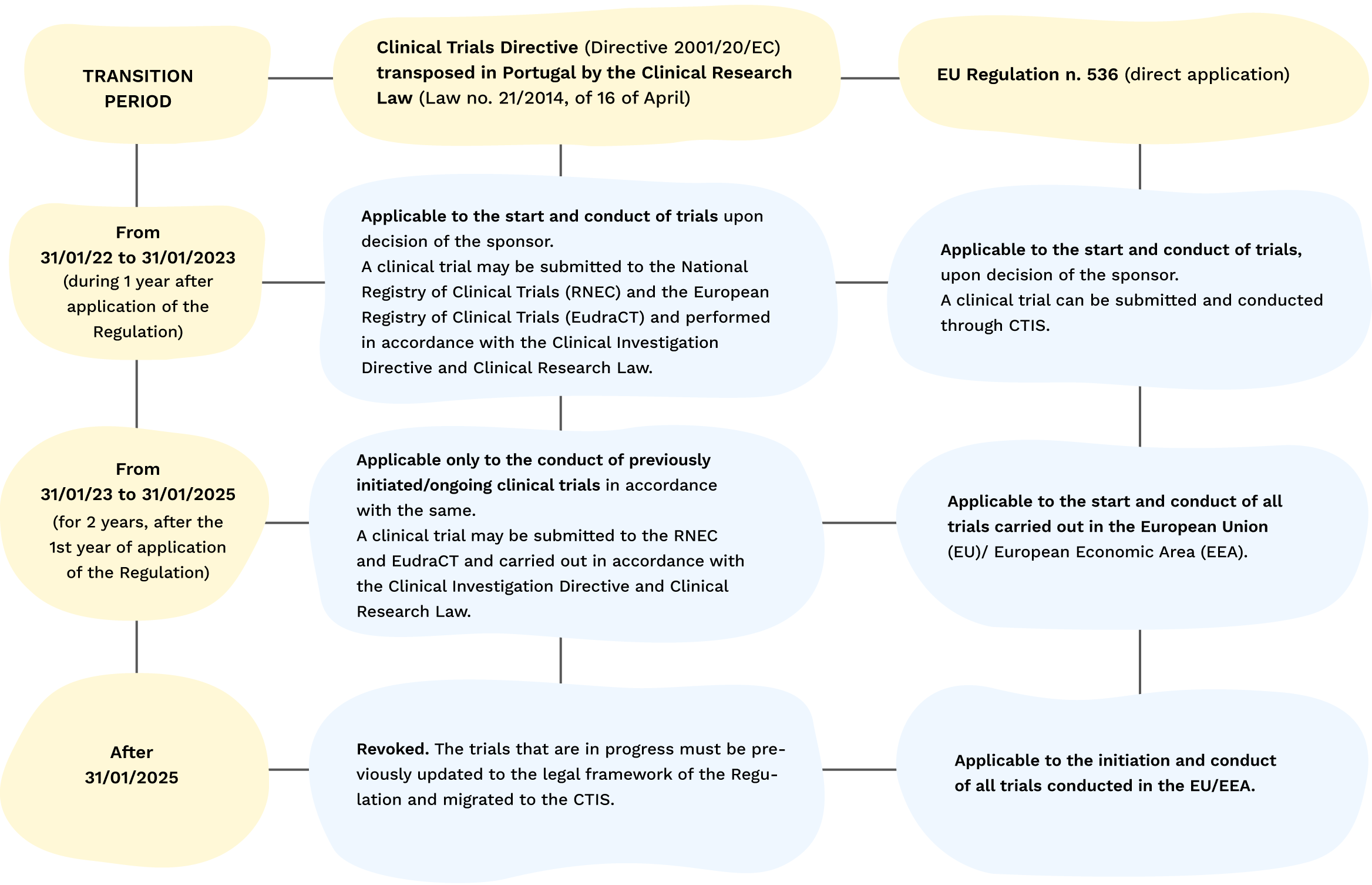
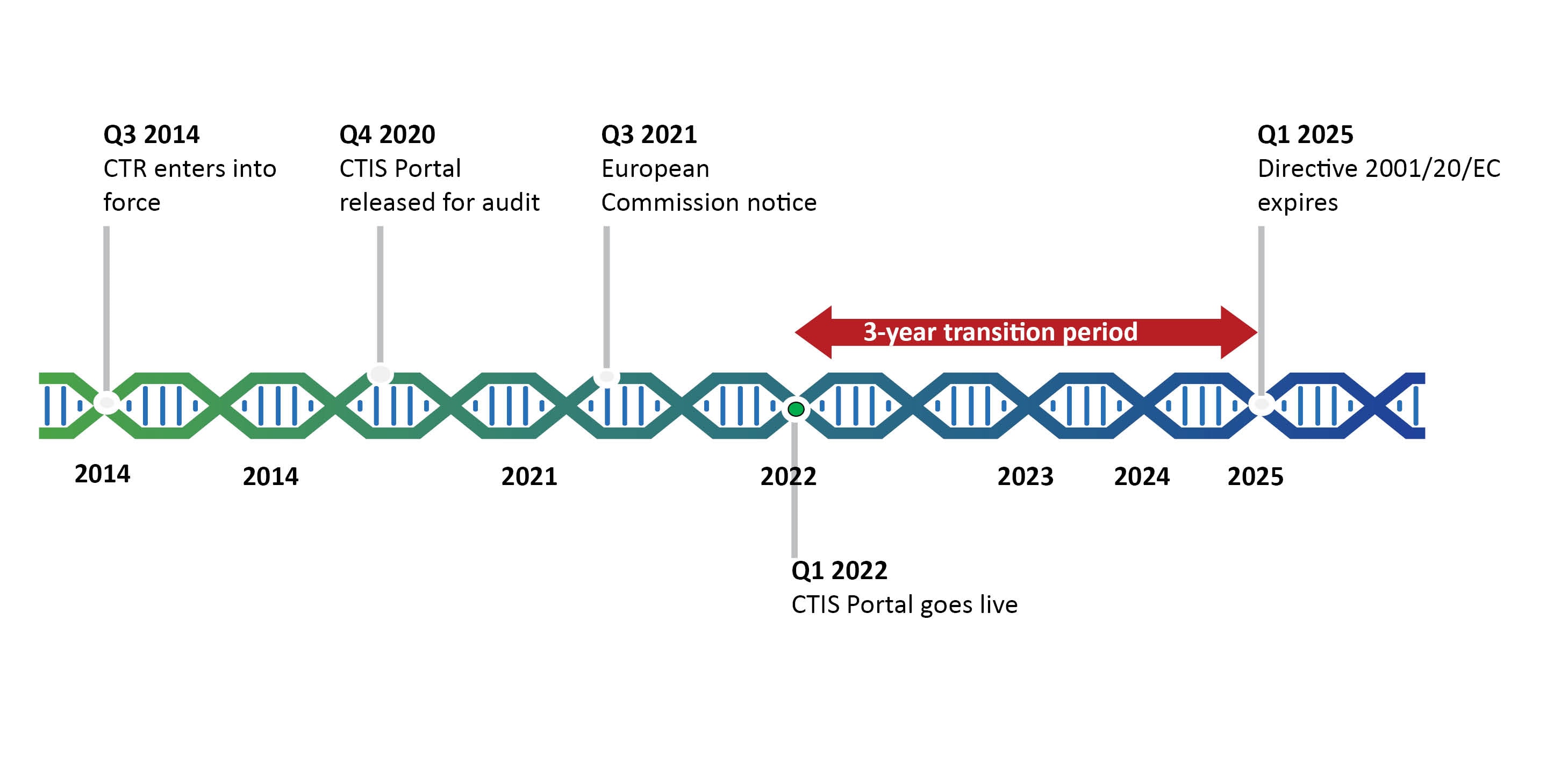
GCP and Quality in “Regulation (EU) 536/2014 on clinical trials on medicinal products for human use and repealing Directive 2001/20/EU” - ScienceDirect
The EU Clinical Trials Regulation: Implications of the New Transparency Rules on Patenting - Lexology

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library

Timeline impact assessment and Revision of Directive 2001/20/EC (see... | Download Scientific Diagram

European Clinical Trial Directive (Directive 2001/20/EC) dr. Cees Smit (NPCF/EGAN) EPF Annual Meeting May 19, Brussels. - ppt download
The Clinical Trials Directive: How Is It Affecting Europe's Noncommercial Research | PLOS Clinical Trials