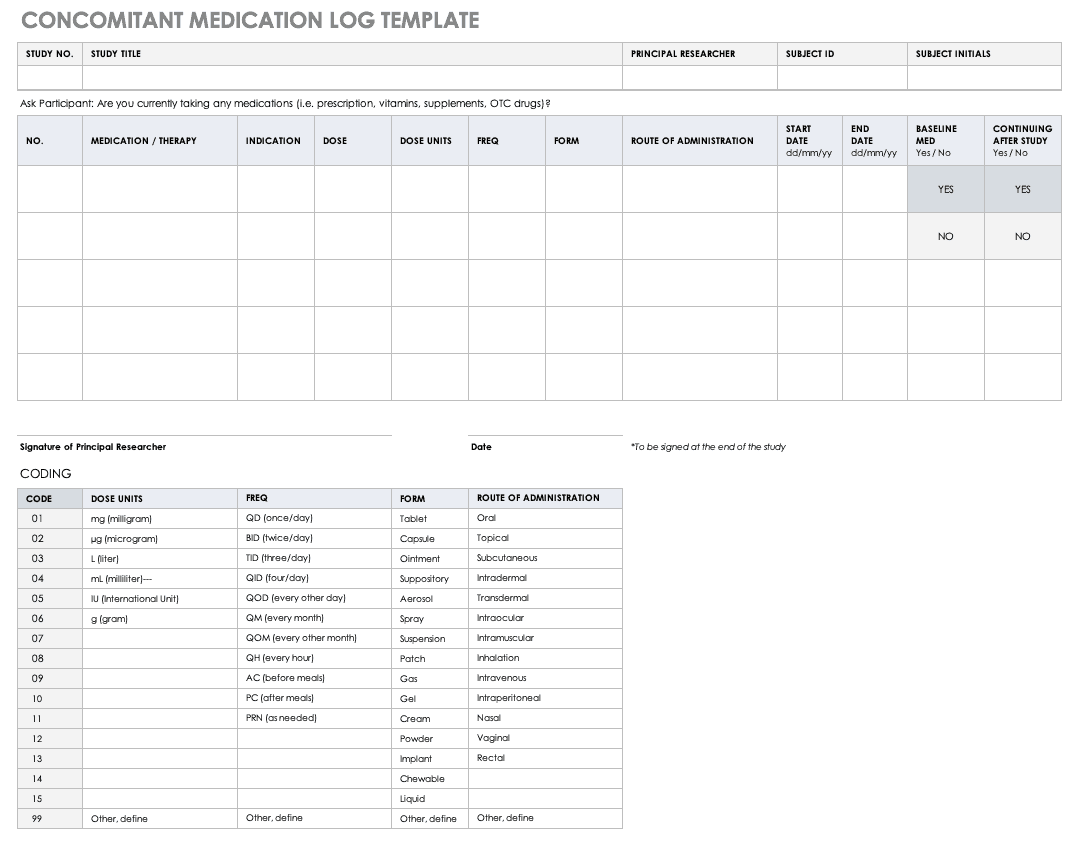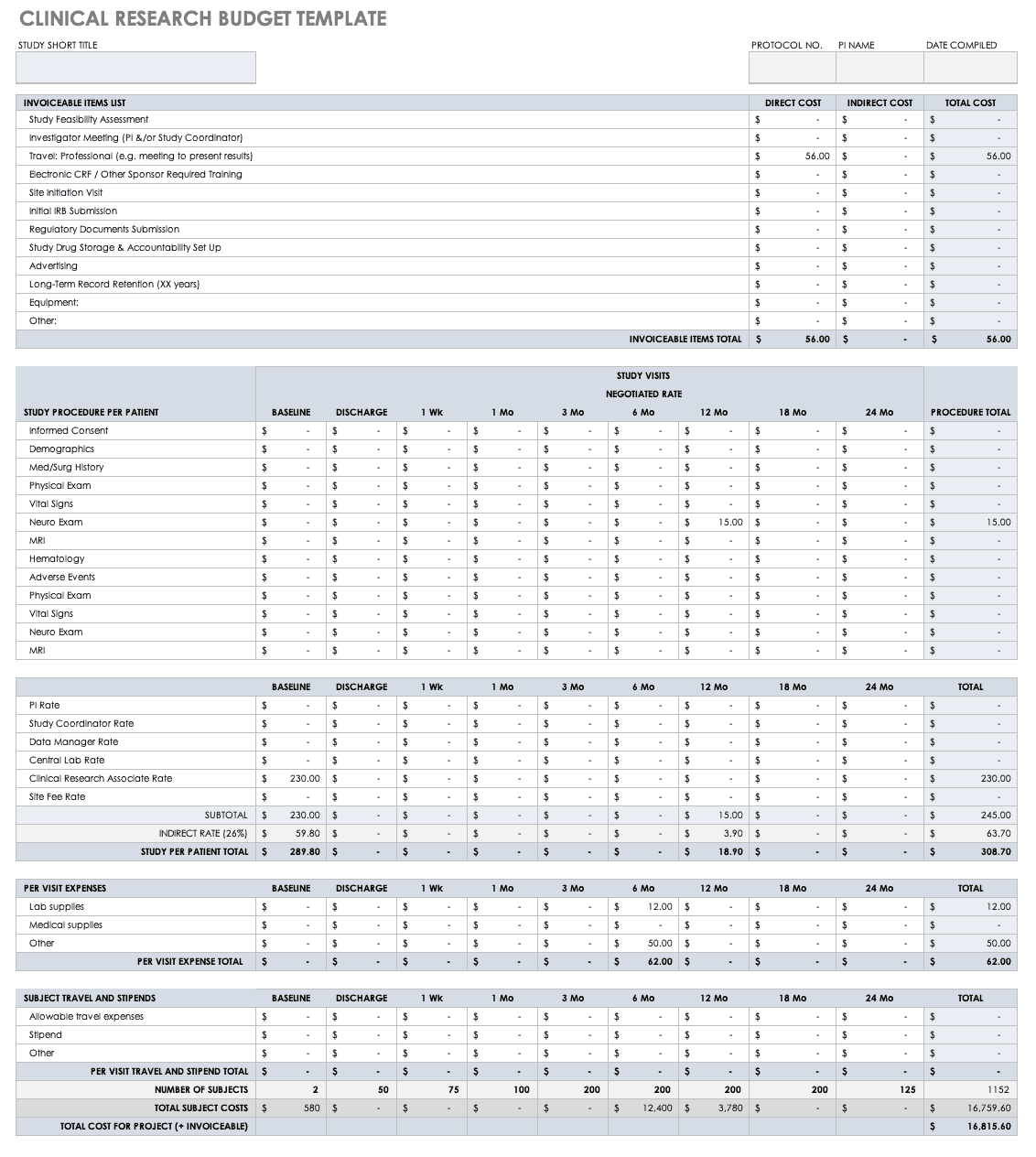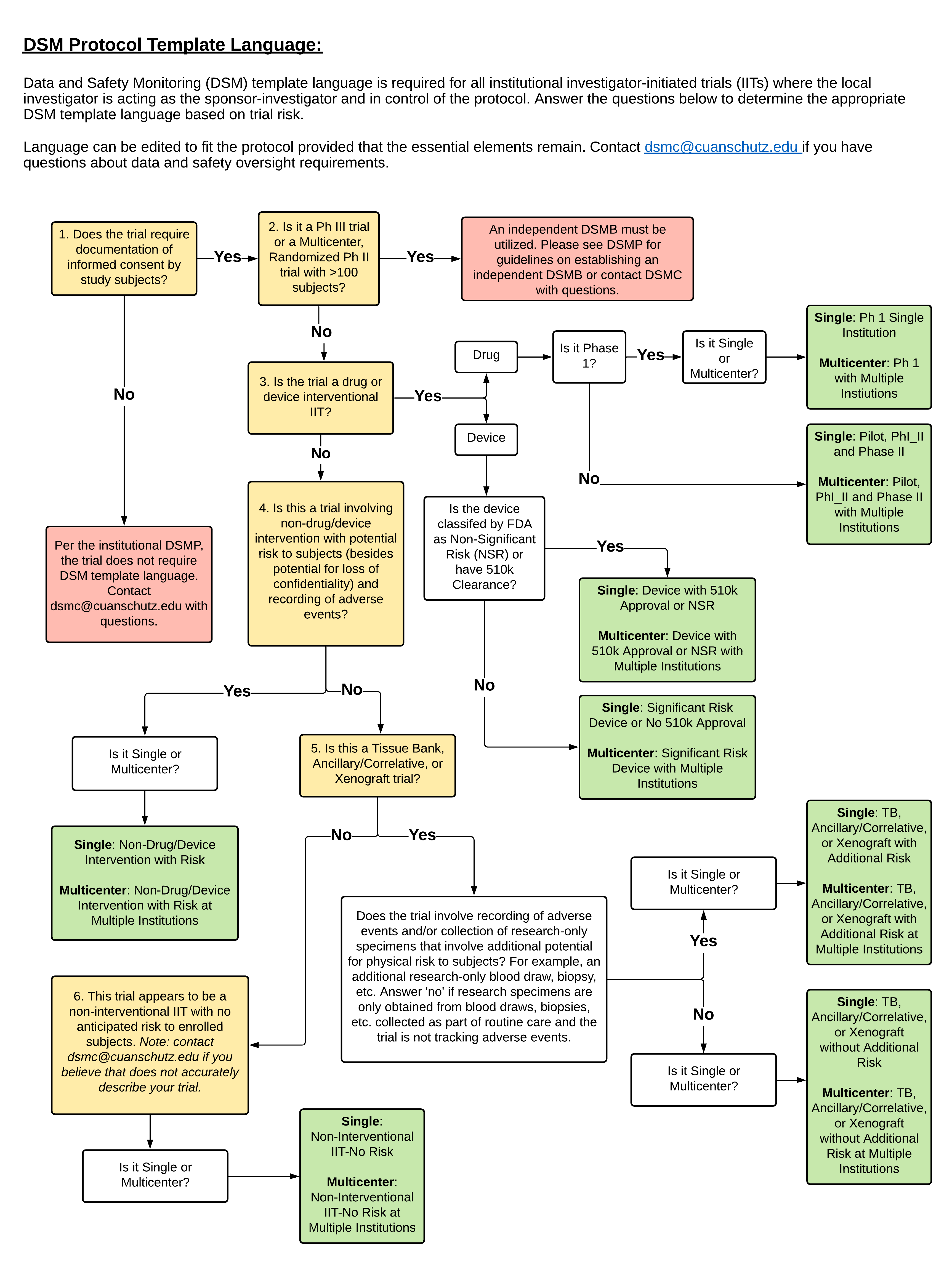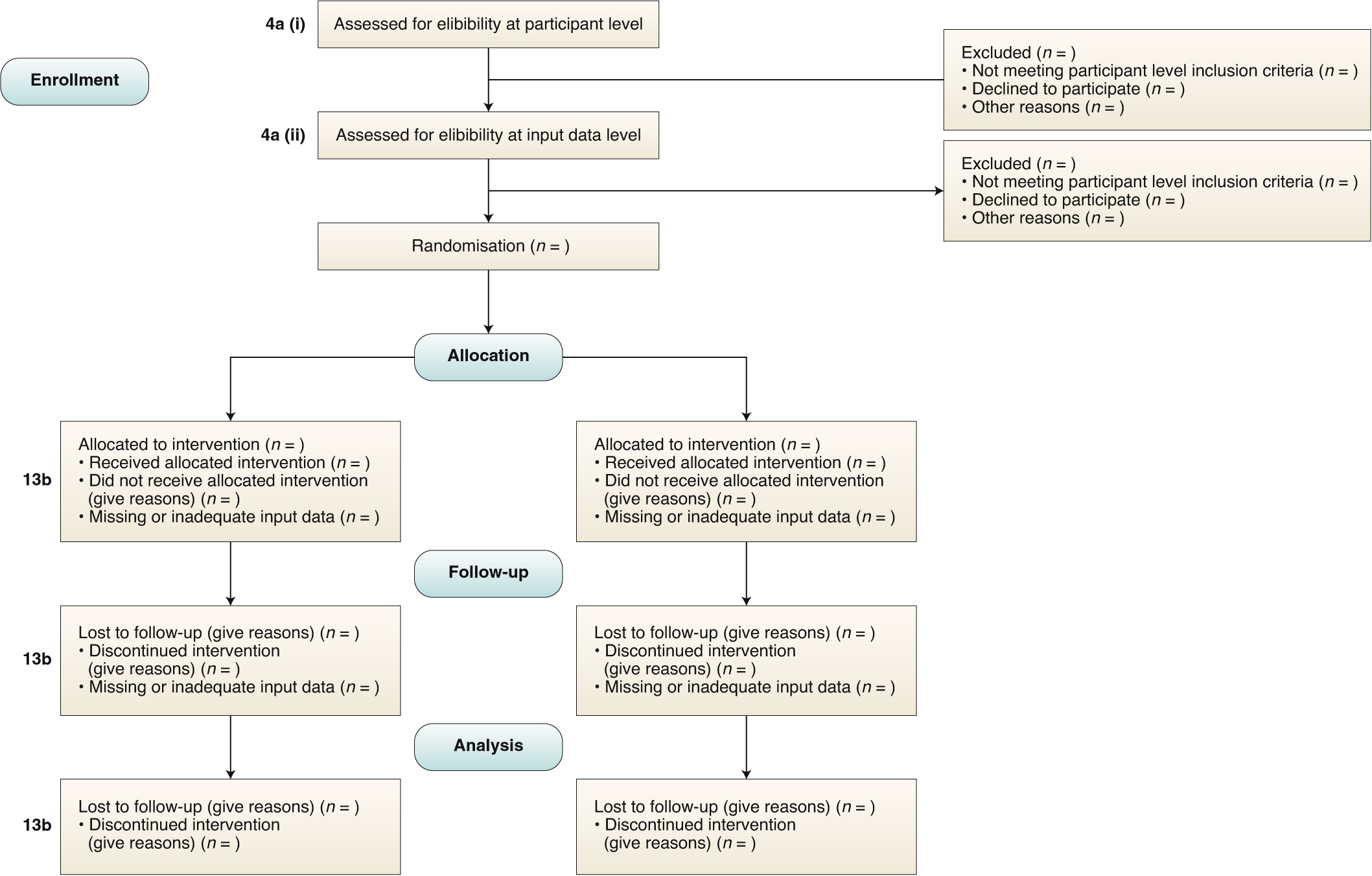
Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension | Nature Medicine
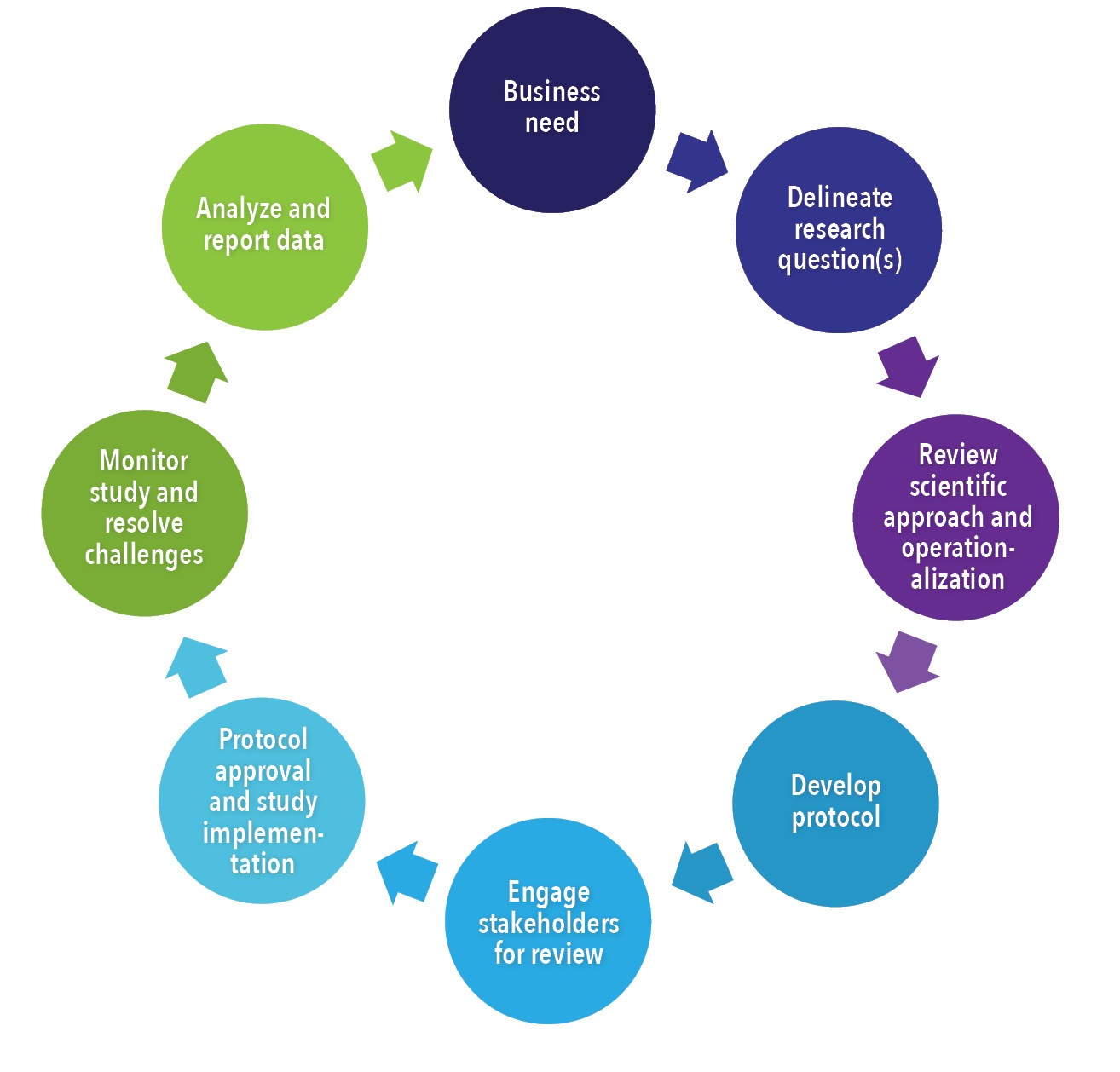
White Paper: Protocol Design in Real-World Evidence: The Indispensable Link Between Strategic Need and Study Execution - Evidera

White Paper: Protocol Design in Real-World Evidence: The Indispensable Link Between Strategic Need and Study Execution - Evidera

STaRT-RWE: structured template for planning and reporting on the implementation of real world evidence studies | The BMJ